
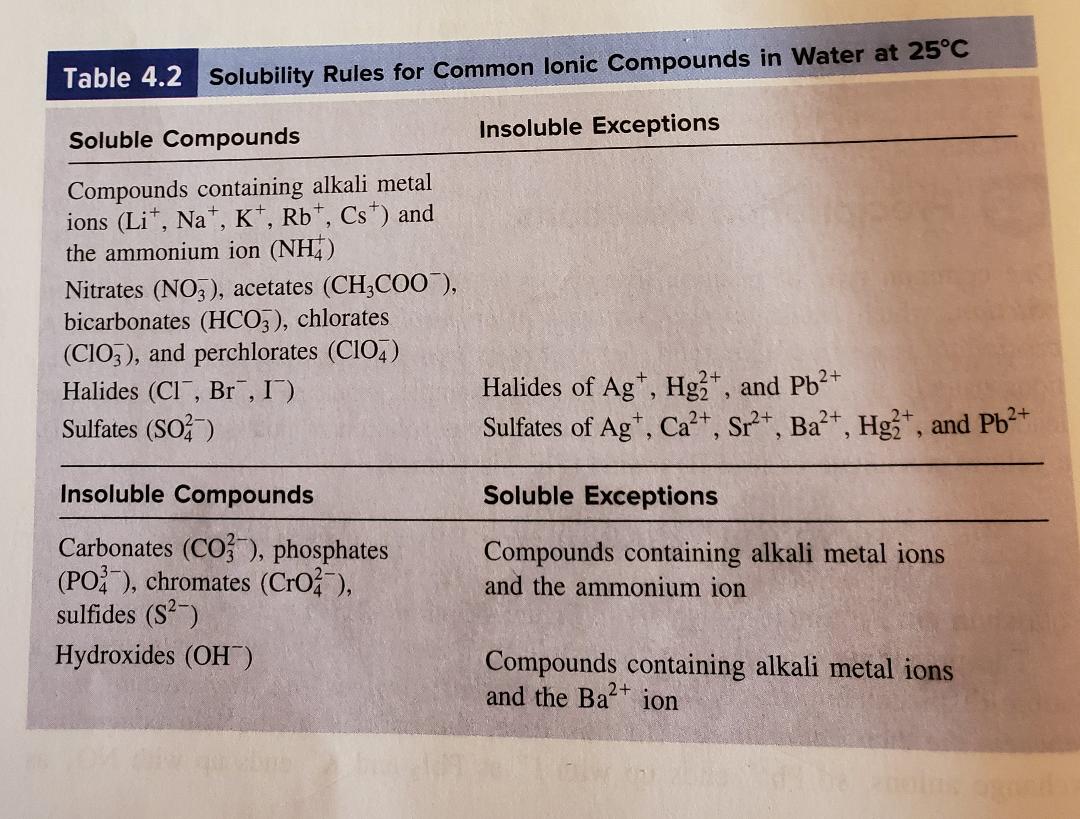
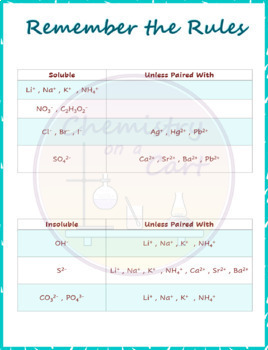
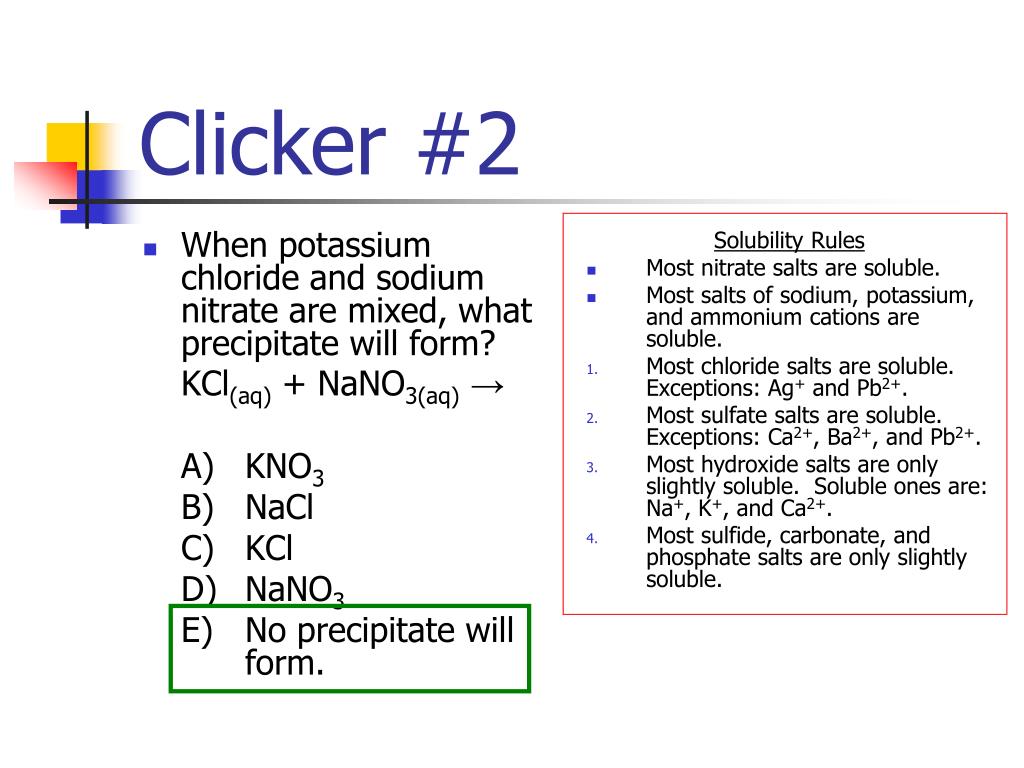
However, these are rather exotic compounds and can be safely ignored at an introductory level. For example, there are some alkali metal compounds that are insoluble. If dilute solutions of both reactants are mixed, so that the concentration of Ag2SO4 does not exceed 0.83g/100mL solution, then no precipitate will occur, and no reaction has occurred. Please be aware that these rules are guidelines only. A common mistake is thinking that (s) is for soluble when instead (s) stands for solid, which would be an insoluble substance that doesn’t dissolve. In like manner, AgCl is insoluble because rule 3 (the smaller) takes precedence over rule 4 (the larger). The product must include a solid product. The reactants are usually two or more ionic aqueous molecules. For example, PbSO 4 is insoluble because rule 3 comes before rule 6. A precipitation reaction will always have a solid product. These rules are to be applied in the order given. The solubility of ionic compounds in aqueous solutions is wide and varied. (6) Sulfates are soluble except for calcium and barium. (5) Carbonates, hydroxides, oxides, phosphates, silicates, and sulfides are insoluble. (4) Chlorides, bromides, and iodides are soluble (3) Silver, lead, and mercury(I) compounds are insoluble. (2) All acetate, perchlorate, chlorate, and nitrate compounds are soluble. (1) All alkali metal (lithium, sodium, potassium, rubidium, and cesium) and ammonium compounds are soluble. Solubility rules that apply to water solution: For the most part, that category has been cast aside. In the past, some teachers would have a third category: slightly soluble. Almost every substance of any importance in chemistry is either much MORE soluble or much LESS soluble. VERY FEW substances have their maximum solubility near to 0.1 M. Any substance that fails to reach 0.1 M is defined to be insoluble. The solubility rules speak to the solubility of salts of cations within a particular group (e.g., group 1 cations) or specific anions. Salts containing the ammonium ion (NH4+) are also. The dividing line between soluble and insoluble is 0.1-molar at 25 ☌.Īny substance that can form 0.1 M or more concentrated is soluble. Salts containing Group I elements are soluble (Li+, Na+, K+, Cs+, Rb+). Isolating the solution and adding a small amount of Na 2CrO 4 solution to it will produce a bright yellow precipitate of PbCrO 4 if Pb 2+ was in the original sample ( Figure 17.11 "The Separation of Metal Ions from Group 1 Using Qualitative Analysis").Īs another example, treating the precipitates from group 1 cations with aqueous ammonia will dissolve any AgCl because Ag + forms a stable complex with ammonia: +.ChemTeam: Solubility Rules General Rules of Solubility
But then again, it wouldnt be really washing a product since youd be only removing the chloride ions and replacing them with whatever anion you had on your added salt. Because PbCl 2 is much more soluble in hot water than are the other two chloride salts, however, adding water to the precipitate and heating the resulting slurry will dissolve any PbCl 2 present. begingroup Hg(I) cations would also precipitate as Hg2Cl2, their low solubility is the whole idea behind Group I of cation analysis.
For example, the precipitated metal chlorides of group 1 cations, containing Ag +, Pb 2+, and Hg 2 2+, are all quite insoluble in water. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Metal ions that precipitate together are separated by various additional techniques, such as forming complex ions, changing the pH of the solution, or increasing the temperature to redissolve some of the solids. Precipitation reactions have been used in general chemistry laboratories (Ricci & Ditzler, 1991), to teach solubility rules (Blake, 2003). As discussed in Chapter 6 "The Structure of Atoms", the other alkali metal ions also give characteristic colors in flame tests, which allows them to be identified if only one is present.

A flame test on another original sample is used to detect sodium, which produces a characteristic bright yellow color. (We cannot use the same sample we used for the first four groups because we added ammonium to that sample in earlier steps.) Any ammonia produced can be detected by either its odor or a litmus paper test. We now take a second sample from the original solution and add a small amount of NaOH to neutralize the ammonium ion and produce NH 3. The only common ions that might remain are any alkali metals (Li +, Na +, K +, Rb +, and Cs +) and ammonium (NH 4 +). At this point, we have removed all the metal ions that form water-insoluble chlorides, sulfides, carbonates, or phosphates.


 0 kommentar(er)
0 kommentar(er)
